Laboratory Standards and Blanks
Reproducibility of 87Sr/86Sr in BCR-2 (basalt), JB-2 (basalt), and JLs (limestone)
Neodymium analyses are normalized to JNdi-1 using 143Nd/144Nd = 0.512115 (Tanaka et al., 2000)
Strontium analyses are normalized to SRM 987 using 87Sr/86Sr = 0.710240
Reproducibility of 143Nd/144Nd in BCR-2
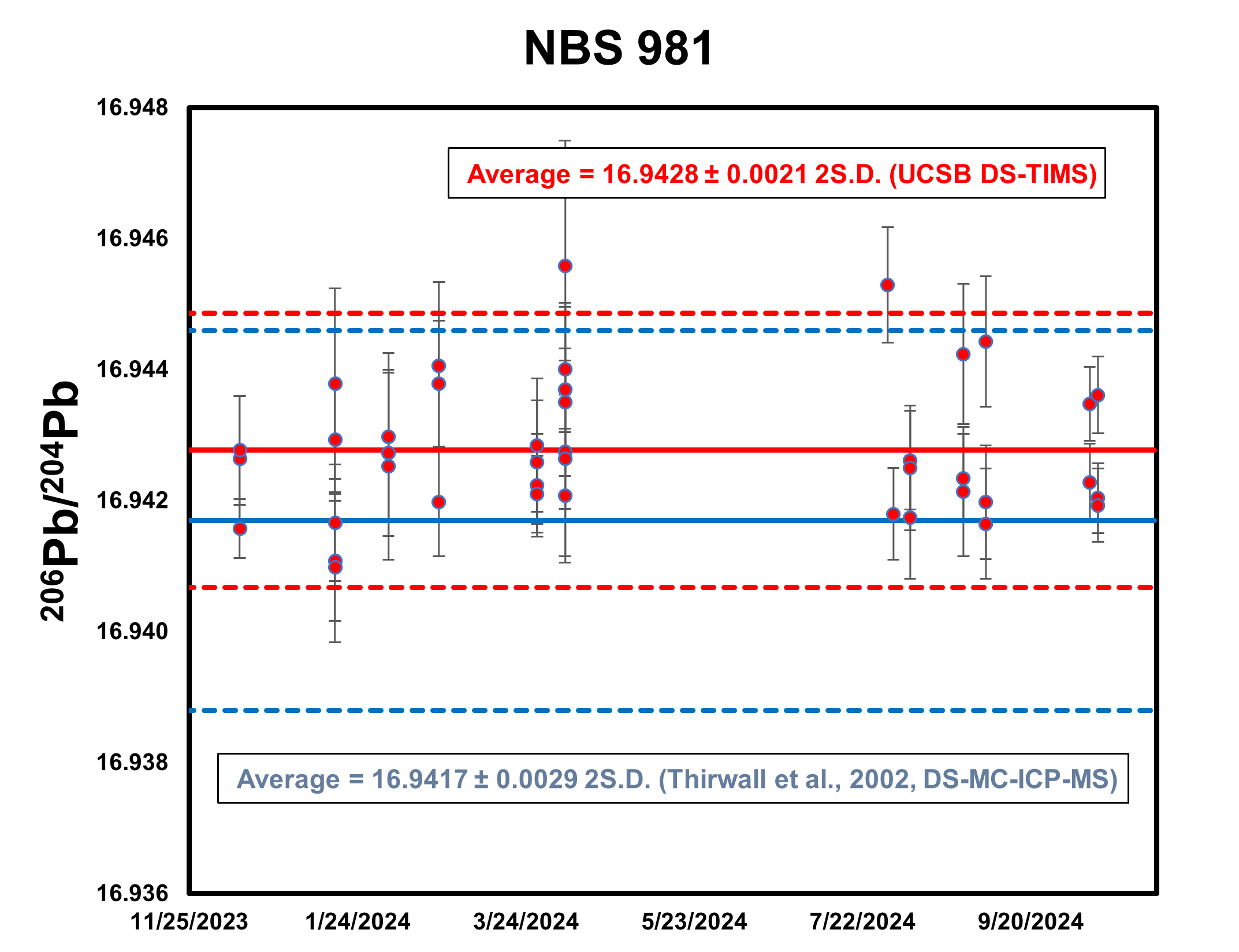
Lead Isotopes
NIST SRM 981 (NBS 981) is a lead (Pb) isotopic standards (along with NBS 982 and NBS 983) developed by the National Bureau of Standards to span the range of Pb isotope compositions found in nature. NBS 981 is the most-widely used standard for lead isotope analysis. A lead isotope analysis cannot be corrected for mass fractionation using internal normalization (unlike Sr and Nd). Therefore, measurements are made using a 207Pb-204Pb double-spike (DS) which enables mass fractionation correction. Replicate analyses of NBS981 by DS-TIMS over a 10 month period yields reproducibility of 124 ppm (2 SD), 142 ppm (2 SD), and 182 ppm (2 SD) for 206Pb/204Pb, 207Pb/204Pb and 208Pb/204Pb, respectively.
Unfortunately, several studies (Baker et al., 2004; Weis et al., 2006; Jweda et al., 2016) have demonstrated that Pb isotopes in BCR-2 are highly variable which is possibly related to Pb contamination during preparation of this standard by the USGS. The JB-2 basalt certified reference material (CRM) developed by the Geological Survey of Japan shows much better reproducibility of Pb isotopes compared to USGS rock standards. Nine analysis of JB-2 (basalt)—from 9 different aliquots of powder dissolved and run through column chemistry separately—in the same turret yielded reproducibility of 164 ppm (2 SD), 129 ppm (2 SD), and 131 ppm (2 SD) on 206Pb/204Pb, 207Pb/204Pb, and 208Pb/204Pb, respectively.
Laboratory Blanks
Full-chemistry blanks — sample dissolution, chemical separation, and filament loading — for basalt samples (~100-200mg) are typically < 50pg for Sr, <15 pg for Nd, and <20pg for Pb. For ultra-low level analyses (i.e. single mineral grain or melt inclusion) blanks are typically <10pg for Sr and <2pg for Nd.